Abstract
HU is licensed in Europe in the prevention of recurrent painful vaso-occlusive crises (VOC) including acute chest syndromes in adults, adolescents and children older than 2 years with sickle-cell disease (SCD).
However, based on US and European expert panel recommendations (Yawn 2014, Habibi 2015) and results from placebo-controlled clinical trials, HU could be useful in SCD patients with severe anemia without VOC since it has been demonstrated to increase total Hb level (Wang 2011) and to decrease the need for blood transfusion.
We hereby present preliminary results on effectiveness and safety data related to the prescription of HU for anemia from ESCORT-HU (European Sickle Cell Disease COhoRT - HydroxyUrea), a multicentric, prospective, non-interventional European study designed to collect long-term safety data on HU in SCD population.
Between January 2009 and June 2017, 1841 patients were enrolled from 63 centers in France, Germany, Greece and Italy, amongst which 126 patients (6.8%) were started on HU for anemia from 34 centers. Of these 126, 96 were HU-naive. These HU-naive patients treated for anemia ('anemic' subpopulation) were selected for analysis to evaluate effectiveness and safety of HU in this indication and compared with data in HU-naive patients treated for other SCD indications.
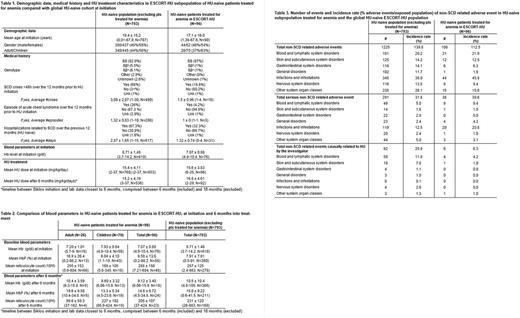
Demographic data and Hb genotypes are displayed in Table 1. The mean age, distribution of gender, Hb genotype and the mean HU dose at initiation were comparable in the 'anemic' subpopulation and the 'non-anemic' HU-naive cohort. Not surprisingly, mean Hb level at initiation was markedly lower in the 'anemic' subpopulation (7.07 ± 0.88 g/dl) than in the 'non-anemic' HU-naive cohort (8.71 ± 1.51 g/dl), with a lower proportion of patients with history of VOC and SCD-related hospitalization prior to HU initiation. The mean HU dose after 6 months was comparable in both groups (15.6 ± 3.83 mg/kg/day and 15.4 ± 4.11 mg/kg/day, respectively).
Variation of blood parameters are displayed in Table 2. Similarly to what has been observed previously, a dramatic rise in Hb concentration (> 2 g/dl) was observed. This increase was comparable in absolute value to the increase observed in non-anemic patients. An increase in HbF was observed in the "anemic" subpopulation, with a near 2-fold increase in %HbF, markedly in children. Changes in reticulocyte counts were inconclusive due to small number of patients in the dataset.
Safety of HU in the population of patients treated for anemia was evaluated by comparing incidence rates of non-SCD related adverse events (AEs) in HU-naive patients treated for anemia with the 'non-anemic' HU-naive ESCORT-HU subpopulation (Table 3). With mean follow-up periods of 18.3 months in 'anemic' subpopulation and 34.2 months in 'non-anemic' HU-naive cohort, preliminary results showed no striking difference in the incidence rate of reported AEs (total and serious) between the two populations (112.5% vs 139.8%, respectively for incidence rate of total AEs), and in the distribution of AEs by System Organ Class (SOC), at least in SOC where the number of adverse events was large enough to allow for comparison between the groups. Similarly, when focusing on AE causally related to HU (as judged by the investigators), the most frequently reported toxicity in the 'anemic' population was myelosuppression (anemia, neutropenia thrombocytopenia, pancytopenia reported in 4 children, one event each), as in the 'non-anemic' HU-naive cohort, with comparable incidence rates.
In conclusion, even though HU is not licensed in Europe in severe chronic anemia, European and US expert panel guidelines recommend treatment with HU in this indication. Data from ESCORT-HU observational study on a subset of SCD patients treated off label in this indication confirmed total Hb level increase while the safety profile of HU in this subpopulation did not differ significantly from the 'non-anemic' HU-naive population.
De Montalembert: Novartis: Consultancy, Honoraria, Research Funding; Addmedica: Consultancy, Honoraria, Research Funding. Brousse: Add Medica: Membership on an entity's Board of Directors or advisory committees. Galacteros: Addmedica: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.